I spent two days with Dr. Jana and Dr. Evelyn Molloy in in their lab, where most project evolve around Molecular Biology workflows to prepare samples to extract biomolecules for HPLC analysis.
We walked the lab from one end to the other, following the natural flow of experiments – from preparing media to handling waste – looking for where they already save resources and where small tweaks could go further.
What I noticed quite quickly was sustainability here wasn’t a “special project.” Many of these practices are embedded in the daily workflow. The trick is recognizing them, optimizing them, and making them part of the lab’s shared culture.
Instead of providing a linear walkthrough, I decided to highlight two main aspects that I think will help you most in understanding sustainable practices: teamwork and creativity.
How they Create A Culture of Sustainability through Teamwork
What stood out most during my walkthrough wasn’t just the practices themselves, but how they reinforce each other to create a lab culture where sustainability is the norm.
The team has found a balance: some things are decided collectively so everyone benefits from a common default, while other choices are left to individuals.
Nevertheless, with Jana and Evelyn as a driving force, they were able to bring about large-scale change that involves several people. Moreover, once sustainable practice is culturally anchored, new lab members and students will automatically adopt these best practices. It’s like a snowball that grows and gathers momentum rolling down the hill – and it was set in motion by just these two individuals.
Alright, let me start where the basics of many of their experiments begin – media preparation.
Agar and yeast extract are bought in bulk and portioned into smaller reusable containers when needed. Simple but effective. Once storage space has been defined, it saves money and generally reduces the risk of not having sufficient supplies.
And once a lab is set up for bulk, it naturally extends to other areas like supplements and buffers. Especially if a staff member or single postdoc is handling procurement and restocking, such a culture shift can make a big difference.
Remaining in the media prep area for a moment, they showed me that Falcon tubes used to prepare vitamins or minerals are washed and reused – before adding them to the media, the contents are sterile-filtered, so reuse doesn’t add risk.

Where possible, glass replaces plastic entirely. The fact that washed tubes and glass bottles are available nearby makes the sustainable option the convenient one, and convenience drives uptake.
Of course, switching to glassware is often only possible if sufficient team members are on board, since autoclaving and cleaning have to be coordinated – but this is what they pulled off. And once implemented, it is one of the best ways to reduce waste while building a new common standard for the entire lab.
Choices made at the system level often help anchor sustainable practices. Jana walked me to the thermocyclers: they are set to idle at 12-14 °C instead of 4 °C. It’s a safe, energy-saving default.
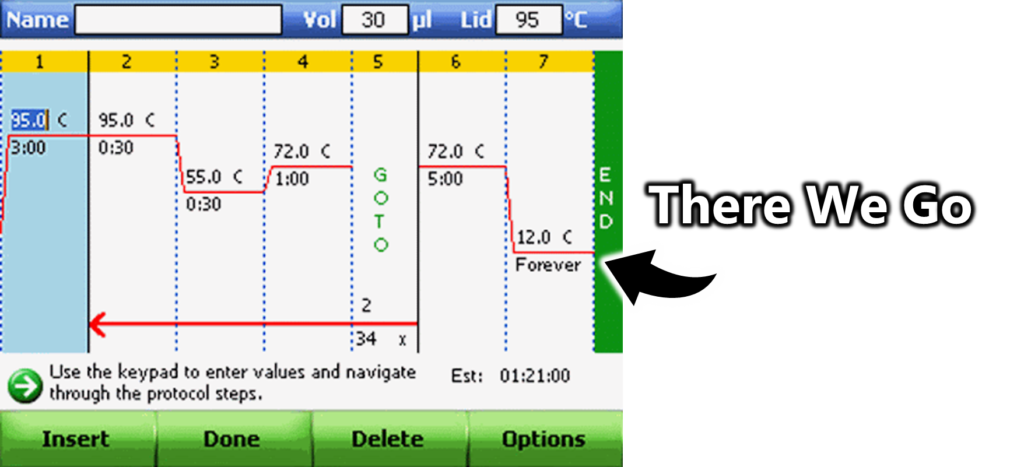
Nobody is prevented from changing it, but very few do. Think about organ donation: if you have to opt in, very few do; if you have to opt out, very few do too. Once the baseline is sustainable, cultural change is much quicker. That means small structural changes can ripple across the whole lab without requiring active effort from each individual.
Then, a really big surprise for me followed, but one that perfectly demonstrates how teamwork saves waste. In their lab, agarose gels are reused until they’re fully consumed. If lanes remain unused, gels are kept in buffer for up to five days so someone else can finish them.

In quick control experiments, gels are even “combed from both sides,” doubling their utility. They know that many have similar conditions to run, so gels (often at 1%) are kept.
One person’s frugality becomes a resource for the next, which saves a whole lot of time for each individual. Gel buffers are swapped roughly once a month, and when evaporation occurs, only water is topped up. These are judgment calls made by individuals, but they fit into the broader culture of using what’s needed and no more.
Energy-saving practices are handled with the same pragmatism. Incubators at 35 °C and 37 °C stay on to ensure constant availability, but shaking functions are turned off when not in use.
This makes sense because the team agrees on what absolutely needs to be running, while individuals are responsible for switching off functions they don’t require. During weekends or holidays, switching those off is often feasible too.
Later, I was accompanied by Evelyn. I noticed that she didn’t wear gloves. She told me that no one really did. They knew which processes were being run, and those didn’t need gloves in that area. Where ethidium bromide is used, areas are clearly indicated, and when hazardous substances are handled, gloves are available nearby.
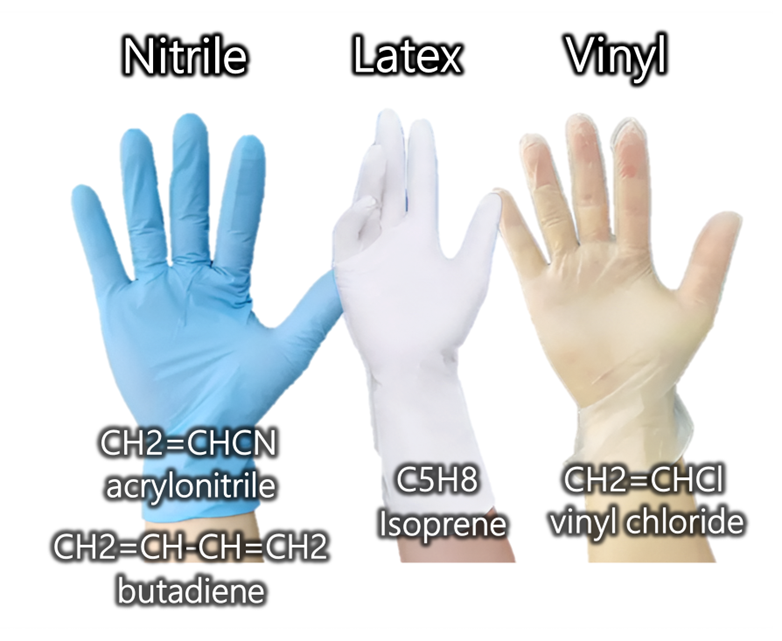
Here, we also find an important benefit of open communication and optimization: where EtBr is handled, students are taught right away- one glove for the handling hand, the other left bare to avoid cross-contamination to tubes or items. The point is that only if you trust your colleagues will you work efficiently as well.
Finally, as mentioned, they took care to leave everyone a personal choice. Again, psychology influences these habits. Glass serological pipettes are stored right next to the plastic ones after autoclaving. Nobody is forced to use glass, but by making the reusable option equally visible and accessible, more people reach for it. And if plastic is ultimately necessary, everybody knows where to find it.

All in all, to my mind, they have shown that establishing a culture around sustainability is extremely powerful – it enables practices that individuals cannot achieve alone, such as a complete switch to glassware, but also nudges better practices like changing PCR holding temperatures.
Nevertheless, to my mind, it is just as important that through establishing such a culture, most will save significant amounts of time, and safety will become a topic openly discussed.
Creativity as a Driver of Sustainable Lab Practice
When we think of creativity in the lab, we normally relate it to cool experiments or interpreting confusing results.
But creativity also plays a crucial role in shaping how we work.
Many of the most effective sustainable practices don’t come from new products or expensive equipment, but from rethinking the basics: asking whether the “standard way” is the only way, and trying something different.
Jana and Evelyn have adopted several really creative approaches. How they go about it? First, by being open-minded and reconsidering whether they could reduce. Second, by asking whether it was safe and, if necessary, coming up with a small test to control for changes.
When I was with Jana, she and her student were about to plate bacteria. Every microbiologist knows the odd frustration of throwing away a sterile plastic spreader after just one use…
Here, they bend the end of a pipette tip instead. It’s sterile, fast, and it avoids an entire category of single-use items.

The same mindset shapes how they prepare competent cells and pick colonies. Glycerol stocks are diluted only to the amount needed -1:10 in most cases, 1:2 – 3 when needed. Toothpicks are used from both ends before disposal.
Tiny changes, yes, but the habit of questioning “Do I really need more?” reinforces itself across hundreds of small actions.
I worked with Jana through an entire PCR workflow. These setups also benefit from this openness to new approaches. If multiple reactions use the same template, DNA is diluted in one well and split across the reactions – reducing pipetting and ensuring equal concentrations.

Mixing samples with loading dye is done on washable mixing plates or parafilm, rather than fresh tubes. Even PCR plates are reused (with silicon lids from Starlab) after thorough cleaning. Many labs would dismiss this outright, but here they tested it, validated it, and adopted it where it works (not for qPCR, but perfectly fine for qualitative checks).
At the gel bench, they challenge one of the strongest “lab dogmas”: one tip per sample. Instead, the same tip is used for 30–60 samples when they only check bands.
With careful rinsing or complete expelling, it works without contamination problems. Again, it’s not carelessness – it’s rethinking whether the old rule was ever necessary for this application. If you have 60 samples, it also saves a lot of time – making both you and your PI happy.
Ligation and assembly kits are another place where imagination pays off. Instead of following the recommended 20 µL reaction volume, they routinely work with 10 µL, sometimes even 5 µL for Gibson or NEBuilder assemblies. Since downstream transformations only use 2 µL anyway, this avoids unnecessary reagent waste. And if it doesn’t work the first time, the smaller reaction makes it easier to simply repeat.
Do I need to mention that your group leader is going to love you for the money you save them?
One reason I was so excited to work with these two was that Evelyn mentioned they reused their electroporation cuvettes. I was intrigued. Admittedly, I only used electroporation during my university years, but I had never heard of someone reusing these cuvettes. Because of their contact with bacteria and the metal included, they are often autoclaved and landfilled, never recycled.

But these two questioned the common narrative. They reuse them more than 30 times with a defined cleaning routine: rinse with water, wash with ethanol, rinse again, rinse twice with deionised water, air-dry, and UV-sterilise for 20 minutes.
Again, this was thought up, tested, and eventually established.
OD cuvettes get different treatment – washed only with water, since ethanol degrades them. But they are reused as well.
Take the filtration station. Instead of just accepting the default, they compared setups. A Steritop filter top weighs 125 g, with a plastic bottle including lid worth 89 g. Both are single-use.
But noticing the waste, they found a filter top in which the filter unit could be switched (purchasing the filters from Merck) while using a normal glass bottle to collect the prepared solution/medium. That means all that is thrown away now is the little filter.

Over a year, that small difference adds up to kilos of plastic saved. It’s not a new invention—it’s looking at the same equipment with a different lens.
I want to highlight once more that safety was always on their minds.
Let’s return to what we talked about already: glove use. They don’t wear gloves for every single step. For ethidium bromide gels, hazardous chemicals, or S2 organisms – yes, gloves are non-negotiable. But for routine E. coli hood work or pouring medium, no gloves. This isn’t about ignoring safety – it’s about applying real risk assessment. The benefits go beyond waste reduction: less skin irritation, better dexterity, and a clearer focus on careful handling.
What ties all of this together is not a single piece of equipment or policy – it’s the willingness to question defaults
Creativity here means asking: Is this rule still valid? Can I do it differently without compromising safety or results? Sometimes the answer is no, and the standard practice remains. But often, the answer is yes—and the result is a lab that saves time, money, and waste while teaching its members to think critically about every step.