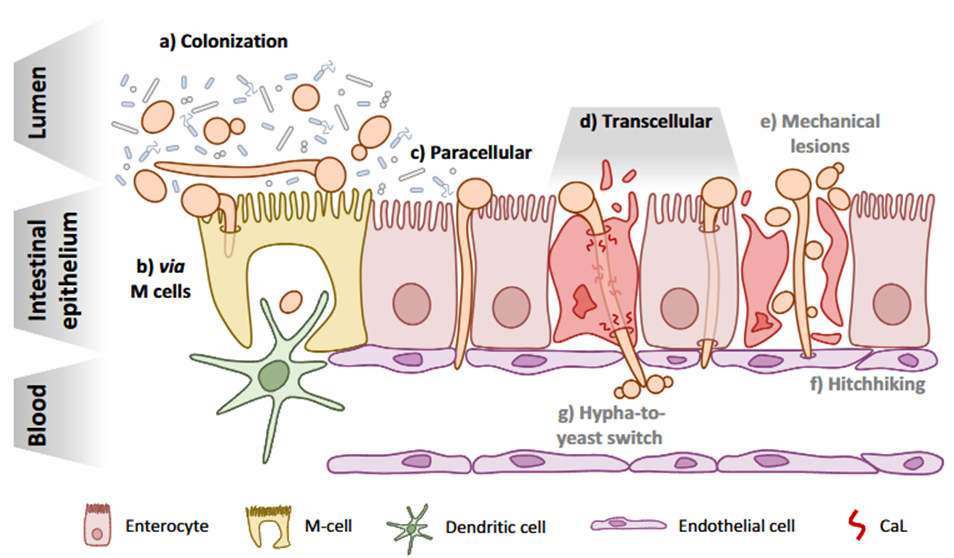
Jakob investigates how different Candida albicans strains cross an intestinal epithelial barrier grown in transwell inserts.
The aim is to test whether the presence of a wild-type, strongly hyphae-forming strain increases the ability of a clinical isolate to translocate and cause damage.
It’s a resource-intensive workflow, involving infection steps, barrier measurements, and potential downstream analyses.
And this was the perfect example to show how experimental design can optimize both data acquisition and environmental footprints.
In other words, this shows that if we optimize our workflows, we often become more sustainable at the same time.
Let’s go through how this looks in practice:
The workflow begins with preparing transwell plates seeded with a 70:30 mix of C2BBe1 and HT29 cells. All you need to know: two intestinal cell lines.
Of note, these transwell inserts allow one to check for barrier integrity since any damage allows particles or in that case fungi/spores to pass through.
Thus, once the epithelial barrier is formed, Candida strains are added alone or in combination. The core of the experiment is to measure barrier integrity.
There are four main ways to measure barrier integrity:
- FITC-Dextran assay: A permeability test where fluorescently labeled dextran (FITC) is added to the top of the epithelial layer. If the barrier is leaky, FITC-Dextran passes through to the bottom chamber. Measuring fluorescence with a plate reader shows how much leakage occurred.
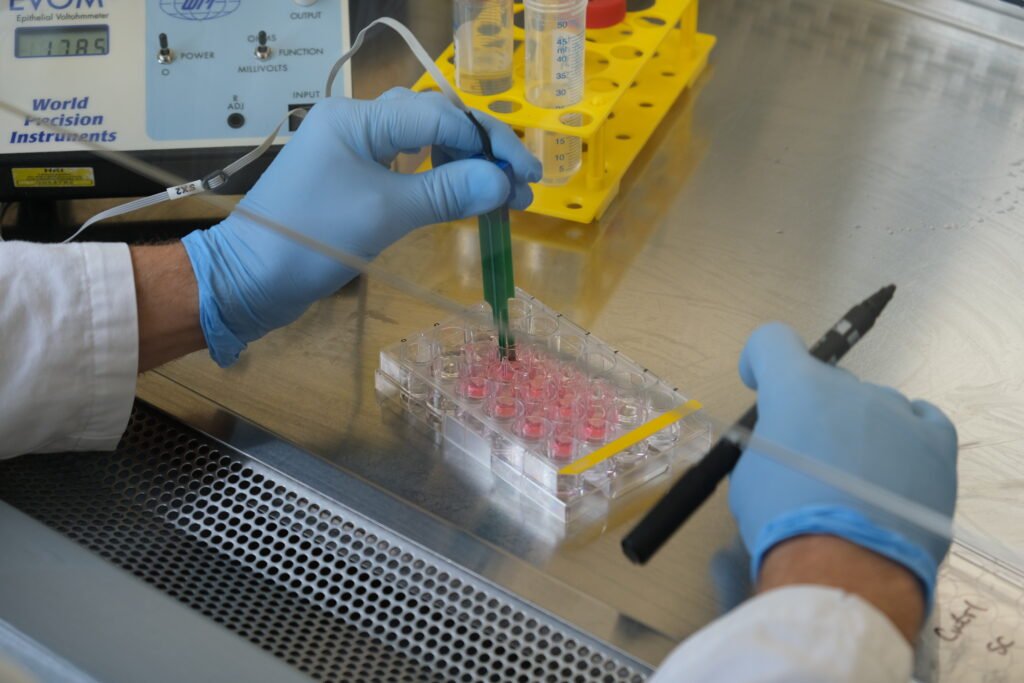
- TEER (Trans-Epithelial Electrical Resistance): A direct, non-invasive method to measure barrier integrity by placing electrodes on both sides of the transwell and recording electrical resistance. A tight, intact barrier gives high resistance; a leaky one gives low. It takes only minutes and doesn’t disturb the cells, so samples can still be used afterward.
- LDH assay: Measures lactate dehydrogenase, an enzyme released into the supernatant when cells are damaged. By comparing LDH levels to a standard curve, researchers can quantify cell death or barrier disruption. It’s a biochemical readout that complements TEER’s functional measurement.
- CFU plating (colony counting): To measure how many Candida cells actually cross the epithelial barrier, samples from the bottom compartment are collected, treated with Zymolyase to break fungal cell walls, and then plated on agar in serial dilutions. After incubation, each colony that grows represents one viable fungal cell (colony-forming unit, CFU). Counting these colonies gives a direct measure of translocation and fungal survival.
Here is what Jakob decided to do:
Instead of relying on FITC-Dextran assays — which are commonly used in other labs as the easy go-to but require specific reagents, extra incubation time, and a plate reader — he chose TEER measurements. TEER can be combined with LDH and CFU plating, is direct, takes only minutes, and doesn’t disturb the cells.
By using TEER first, he avoids an additional incubation step, cuts reagent costs (and their environmental impact), and eliminates the plasticware that would have been needed for FITC-Dextran. And because TEER leaves the cells intact, he collects supernatants for LDH assays or other readouts.
Why not run FITC and LDH together? For LDH assays, they collect 50 µL of supernatant from each transwell insert – half of what’s available – to avoid disturbing the barrier. This means LDH and FITC-Dextran can’t be run from the same well in the same experiment.
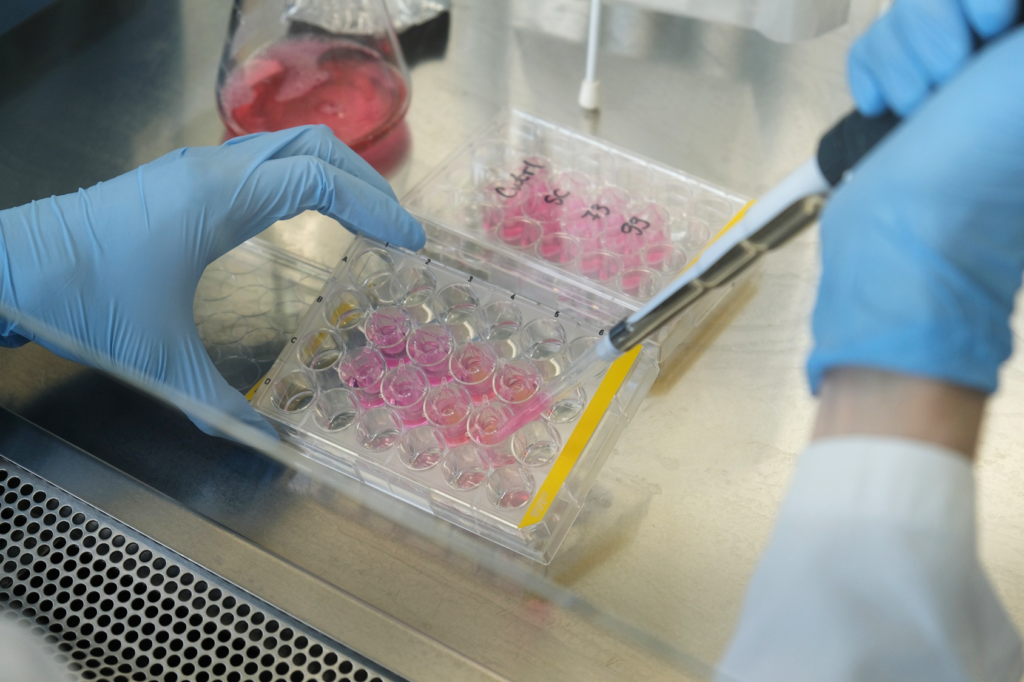
Colony-forming unit (CFU) plating requires Zymolyase digestion — a two-hour step. The team avoids combining this with FITC-Dextran, which would extend the experiment by another hour and require extra consumables. Instead, they keep workflows separate unless both readouts are absolutely essential.
For us, this seems obvious, but it requires taking a step back and deciding which assays to prioritize. Once again, optimizing data acquisition and quality leads to the same conclusion as sustainability considerations. The critical step is to sit down and search for alternative methods at the level of experimental design.
But experimental design stretches beyond methodology. Jakob also looked closely at the design of single-use items and pipetting orders.
For example, instead of using 1.5 mL conical-bottom tubes for washes, they work with flat-bottom 2 mL tubes. The flat base allows pouring off washing medium without disturbing the pellet, meaning no pipette tip is needed. The same shape also makes vortexing more effective for resuspension.
What looks like a small change eliminates several plastic tips per wash step and saves time at the bench.

In LDH assays, they reuse a pipette tip for both technical replicates when handling low-risk reagents like stop solution, cutting tip use in half for that step. Reservoirs for non-hazardous solutions are labeled, rinsed, and reused rather than discarded after one use.
At the same time, he is careful to use fresh tips for dilution series, pipetting from higher to lower concentration to avoid cross-contamination — again showing that sustainability never overrides data integrity.
Moving to CFU plating, technical replicates are plated in duplicate for the same reason. This doubles the number of plates, but it catches variability early and prevents entire experiments from being repeated. Supernatants are also kept in the fridge for short periods in case re-plating is needed — another safeguard against wasting days of work and materials.
Sometimes experimental design cannot optimize sustainability. In his plate layouts, Jakob avoids the outer wells because, due to temperature differences and humidity variation, medium evaporates faster and cells grow more slowly.
That also means differentiation takes longer. This would disrupt workflows. If possible, the number of replicates is adapted; otherwise, empty wells are accepted as an unfortunate reality.
Finally, a few smart tweaks stand out. For cleaning the electrode during TEER measurements, they keep two 50 mL tubes — one for PBS, one for ethanol — and reuse them for up to a month. Ethanol volume is kept slightly lower so it never contaminates the PBS.